Relief as treatment for rare condition is extended
 BBC
BBCA mother says she and her family can "live again" after access to her son's life-enhancing treatment for a rare genetic disorder was extended.
NHS access to the drug Brineura, which slows the progress of Batten disease, was set to end this month but NICE and NHS England have now come to new agreement with the drug's maker.
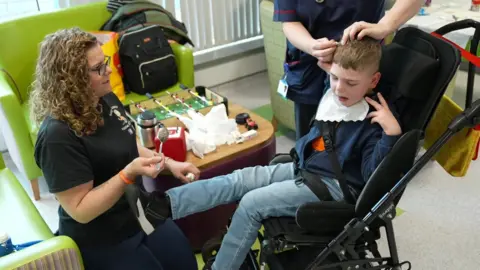
One of those who receives it is Isaac, eight, who has CLN2 Batten disease, which was diagnosed in August 2021.
His mother Aimee Tilley, from Kettering in Northamptonshire, said: "We know it's not a cure, we still see regression, but it's a huge amount slower, so he's gaining years, not just days or weeks."
Batten disease, a rare genetic disorder, causes a rapid decline in a child's ability to walk, talk and see, and is estimated to affect about 40 children in the UK - with an average life expectancy of about 10 years.
Brineura is the only approved treatment that slows the condition's progress.

The new agreement will mean those on the drug, and those who start the treatment before the end of the year, can receive it on a permanent basis.
Ms Tilley said: "We are extremely relieved that Isaac is going to continue to have this treatment.
"This black cloud that we've had hanging over us has gone. We feel like we can live again."
'We have won this battle'
NICE said it and NHS England would continue to work with BioMarin, which makes the drug, on "a solution to secure access to all future patients but at the moment the treatment is not considered cost effective".
Ms Tilley says her family "will not stop fighting for the children of the future".
She said: "They deserve it just as much as the children now and we have won this battle, but we will win the war."
Ms Tilley said Isaac was "having seizures, losing his mobility, he can still walk with a walker or walk holding our hands [and] he has now gone blind".
But, she added: "He's happy. He still enjoys theme parks, going horse riding and he still does a lot of things that children of his age can do we just have to adapt them for him."

Helen Knight, director of medicines evaluation at NICE, said she was "pleased" an agreement had been reached.
She added that NICE and NHS England remained "committed to working with the company to try to reach a long-term deal that will give access to [Brineura] to all eligible people" after December.
Follow Northamptonshire news on BBC Sounds, Facebook, Instagram and X.
